Original publication time: 2021.01.19
Original publication journal: Journal of Materials Science & Technology
(DOI: 10.1016/j.jmst.2020.12.013)
Article content:
On January 16, 2021, the Journal of Materials Science & Technology published an academic article from Yanshan University entitled "Effect on microstructure and plastic deformation behavior of a Zr-based amorphous alloy by cooling rate control". They prepared Zr41.2Ti13.8Cu12.5Ni10Be22.5 amorphous alloy samples with the same diameter (8mm) using self-designed molds with different cooling capacities (refractory steel, pure graphite and copper molds). In addition, by eliminating the size effect, the effect of cooling rate on the microstructure and compressive deformation behavior of Zr41.2Ti13.8Cu12.5Ni10Be22.5 amorphous alloy was investigated. Analysis of the cooling curves showed that the instantaneous cooling rates of the alloy melts at the glass transition temperature (Tg) of the heat-resistant steel, pure graphite and copper molds were 45, 52 and 64 K·s - 1, respectively. X-ray diffraction, differential scanning calorimetry and high-resolution transmission electron microscopy analysis showed that with the decrease of cooling rate, a small amount of icosahedral atomic clusters and nanocrystals appeared in the local area of the amorphous alloy, and the free volume increased with the icosahedral atomic clusters. decreased as the number of clusters and nanocrystals increased. Compression test results show that the elastic strain, yield strength and compressive strength of amorphous alloys slightly change with decreasing cooling rate, while the plastic strain increases gradually. By fitting, the effective size-vessel-like mode is linearly related to the enthalpy released upon structural relaxation and plastic strain, indicating that at lower cooling rates, trace nanocrystals in amorphous alloys cannot effectively improve their plasticity and free volume and mainly affects its plasticity.
Experimental materials and methods:
Alloy ingots with composition Zr41.2 Ti13.8 Cu12.5 Ni10 Be22.5 were prepared by arc melting a mixture of pure Zr, Ti, Cu, Ni (≥99.9%) and Be (≥99%).
Its amorphous structure was characterized by X-ray diffractometer (D/max2500/PC, CuK radiation) and thermal analysis was performed by Netzsch STA449C differential scanning calorimeter (DSC) under argon flow. And the TEM (JρEOL-2010) image of the microstructure can be recorded by increasing the temperature from room temperature to 1200 K at a heating rate of 20 K·min-1. Compression performance tests were performed on compression specimens with a diameter of 2 mm and a length of 4 mm at a strain rate of 5 × 10 - 4 s - 1 using an Instron 5982 testing machine. To ensure the accuracy of the results, the compression test was repeated at least three times. The fracture morphology of the compressed samples was observed by scanning electron microscope (Hitachi S-3400). The mean effective size of the vein pattern was statistically measured by the linear intercept method.
Observation by scanning electron microscope showed that the shear band density gradually increased with the order of heat-resistant steel mold, pure graphite mold, and copper mold (see Figure 1 for the SEM image of the sample port).
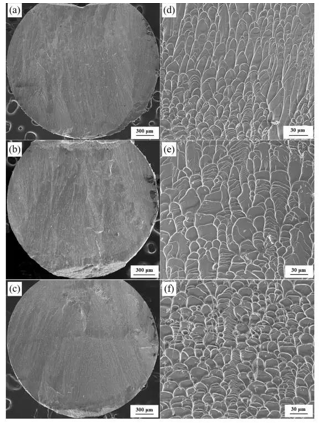
figure 1
Different mold materials exhibit different cooling capabilities. The cooling capacity of the mold can be characterized by the heat storage coefficient (b), which is expressed as b=picture (λ, c, and ρ represent thermal conductivity, specific heat capacity, and density, respectively). The calculation results show that the b value increases gradually in the order of heat-resistant steel, pure graphite, and copper mold, which is consistent with the cooling curve result (Fig. 2).
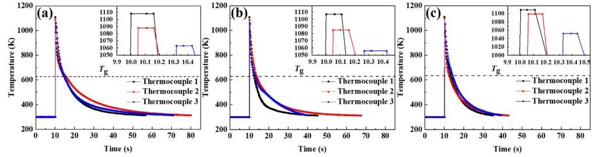
图2
To determine the plastic strengthening mechanism, the effective size of the vein diagram was fitted to the plastic strain and free volume values. Statistically measure the effective size of the compressive fracture of the vein pattern of the different cast specimens. The effective vein size of heat-resistant steel, pure graphite and copper casting samples is 11.5, 9.1 and 7.8 μm, respectively, indicating that the effective size of vein pattern decreases with the increase of cooling rate. Figure 3 shows the glass transition before expanding the DSC curve: the enthalpy changes of relaxation are calculated to be -0.6, -2.8, and -5.3 J g-1 for the samples cast from heat-resistant steel, pure graphite and copper molds, indicating an increase in free volume, respectively The number of increases with the cooling rate.
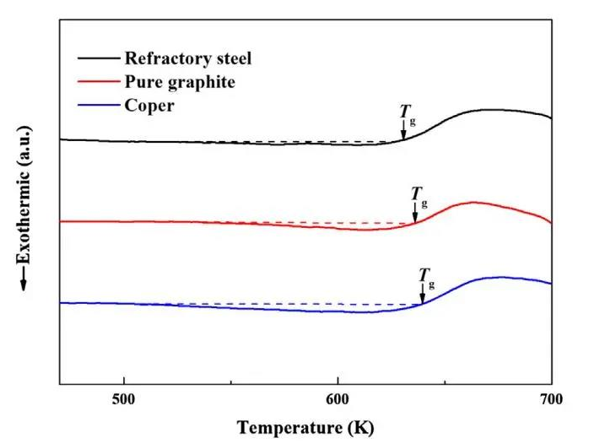
image 3
in conclusion:
The cooling rate curves of amorphous alloy melts under different molds are all in line with the ExpDec2 function model. The cooling rates of amorphous alloys at the glass transition temperature (Tg) of heat-resistant steel, pure graphite and copper molds are 45, 52 and 64 K·s-1, respectively.
With the increase of cooling rate, the yield strength, maximum compressive strength and elastic strain of amorphous alloys did not change much, while the plastic strain increased gradually.
The effective size of the vein pattern of the specimens cast with heat-resistant steel, pure graphite and copper molds were 11.5, 9.1 and 7.8 μm, respectively, indicating that the effective size of the vein pattern decreased with the increase of cooling rate.
The relaxation enthalpies of heat-resistant steel, pure graphite and copper molds were calculated to be -0.6, -2.8 and -5.3 J·g - 1, respectively, indicating that the amount of free volume increases with the increase of cooling rate.
As the cooling rate decreases, the atomic arrangement order of the amorphous alloy increases, and a small amount of crystalline phase is observed. However, due to the extremely small number of crystalline phases, the plasticity cannot be effectively improved.
Interpretation and analysis of this article:
It is generally believed that the softening process of amorphous alloys is related to the formation of shear bands, that is, the material is often accompanied by the formation of shear bands during the deformation process. Amorphous strength. In this process, a large amount of heat energy is released to soften the amorphous, and at the same time, only one or a few main shear bands are activated in a certain direction, so that the amorphous is deformed locally and fractured along the direction of the main shear band. lead to poor plasticity.
During the cooling process, there is theoretically a relaxation process at any temperature. The relaxation process is accompanied by the annihilation of the free volume, and at the same time makes the model transition from a certain equilibrium state to a new equilibrium state. The relaxation time generally increases by an order of magnitude for every few degrees lower in temperature. At a certain temperature, if the relaxation time of the model is so long that the recovery process of the equilibrium state cannot keep up with the cooling process, that is, the model will enter the next non-equilibrium state before it can relax, and a glass transition will occur to form an amorphous state.




 CH
CH





